Finding a viable treatment for Alzheimer’s disease (AD) continues to frustrate researchers around the world. The University of Minnesota Center for Drug Design’s Robert Vince, Ph.D., and Swati More, Ph.D., aren’t exempt.
Vince, More, and their colleagues are developing a drug to treat early AD, but they wanted a faster way to test whether their compound was working. So they created a cost-effective, noninvasive eye scanning technology to do the job, and their breakthrough is commanding international attention.
“Right now, there are no successful drugs available to treat AD,” says Vince, a professor in the College of Pharmacy and director of the CDD, “and it doesn’t seem reversible. So there’s a consensus that we should try to intervene early. But how do you identify the disease before someone starts losing cognitive function? This retinal scan is one such test.”
Focus on the eye
The primary mission of the CDD is to make new drugs for various diseases. In fact, Vince was instrumental in developing an AIDS drug that, through licensing, has brought more than $600 million in royalties into the University. Those dollars now help fund the creation of new drugs and support work that’s moved beyond antivirals. Additional philanthropic support for Vince and More’s work on the retinal scanner came from the Vince Family Fund for Alzheimer’s and Neurological Disease Research and the Wallin Neuroscience Discovery Fund.
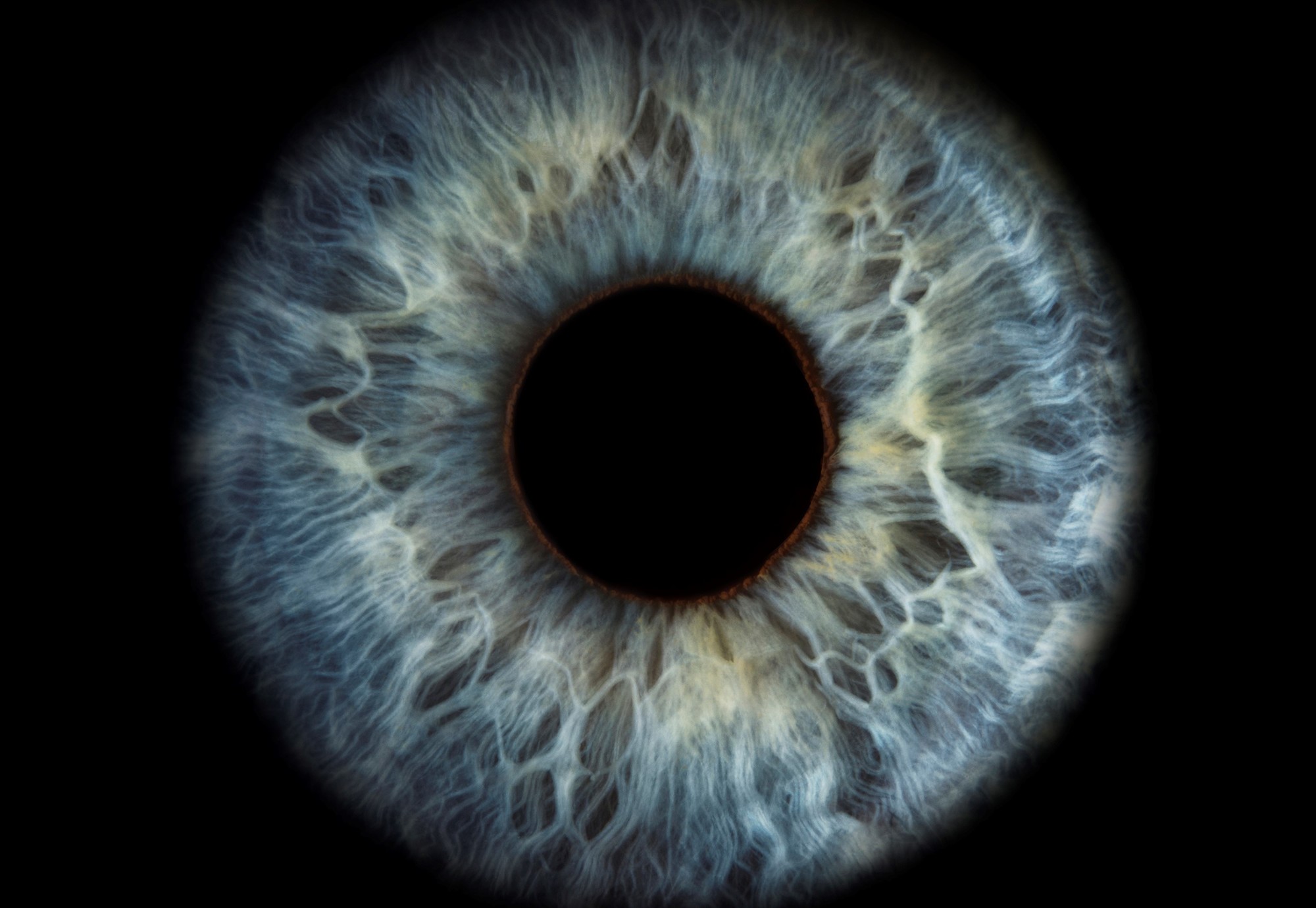
As Vince and More, an associate professor at the CDD, worked on a way to find out whether their AD drug was viable, they turned to a hyperspectral imaging (HSI) microscope to look for signs of AD in brain cells. When that worked, they switched their attention to the retina, which shares the same neurons as the brain.
Vince and More thought that if people were developing AD’s signature amyloid beta protein plaques in their brains, biomarkers for those plaques should be visible in the retina, too. Using the HSI microscope, they examined eye tissue, first from healthy mice and mice modeling human AD, and later comparing eye tissue from people who had died of AD and those who had died of other causes. Their theory was confirmed.
“But the big question remained: Could we identify the earliest stage of AD in a living human, when biochemical changes in the brain are just beginning, by scanning the retina?” More says. “We needed a new scanner to test the theory.”
They approached the company that manufactures the microscope to help them develop a camera. With that first, one-of-a-kind retinal scanner, the team completed a small preclinical study involving 20 people who had AD and 16 healthy individuals—and confirmed that they could indeed identify signatures of the protein that eventually forms AD plaques.
Quick, painless, and inexpensive
The CDD has licensed its retinal scanning technology to the Canadian company RetiSpec, which is launching clinical tests at five locations. So far, the team has completed retinal scans of about 30 individuals and plans to evaluate 100 total.
In addition to being noninvasive and inexpensive, the scan itself takes less than 10 minutes, Vince says. He hopes the retinal scan could one day become part of an annual eye exam.
The new camera also can be used to help researchers track the progress of other promising early-intervention drugs. U investigators are starting to explore whether the technology could be useful for diagnosing other neurologic conditions, such as Parkinson’s or Huntington’s disease, sooner, too.
While early diagnosis is important, physicians still need better drugs to treat Alzheimer’s disease. Today, More says, lifestyle changes such as physical activity, diet modifications, and brain exercises are the best available interventions for people found to be at higher risk for developing the disease. And that makes the CDD team’s work to develop a viable drug—and quickly—even more important.
“Developing a drug today, from discovery to approval, costs about $1.5 billion, so you want to make sure your compound is the best possible one,” says Vince. “This retinal scanner can help us modify the compounds that we, and other researchers, develop to ensure they’re working.”


